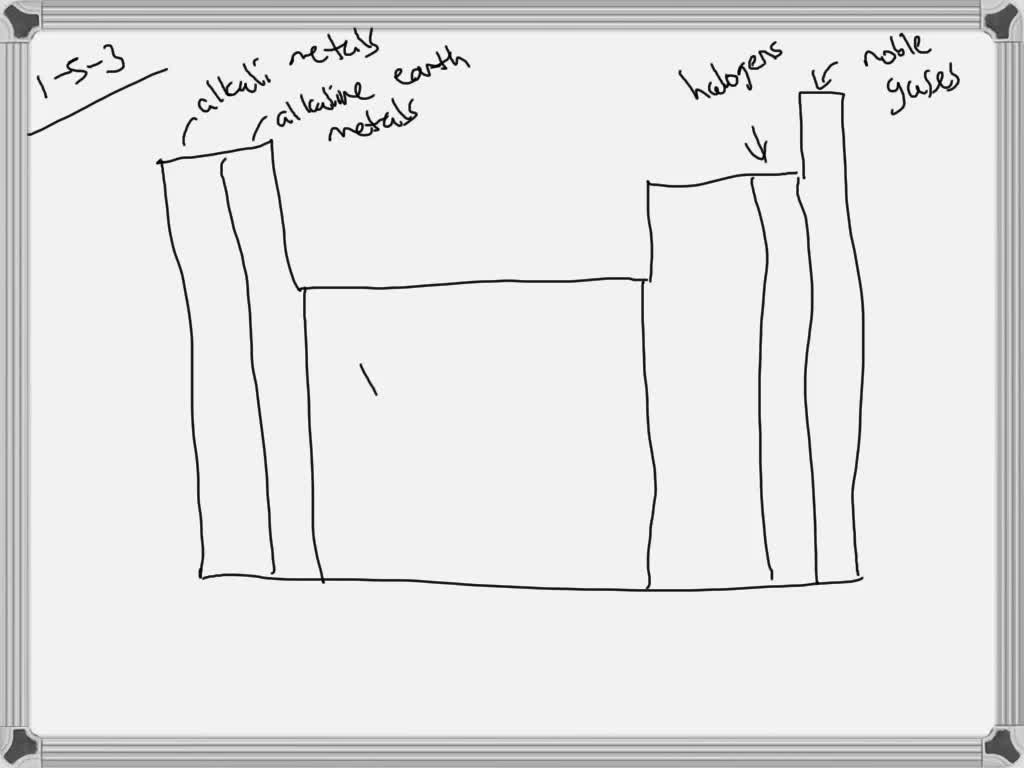
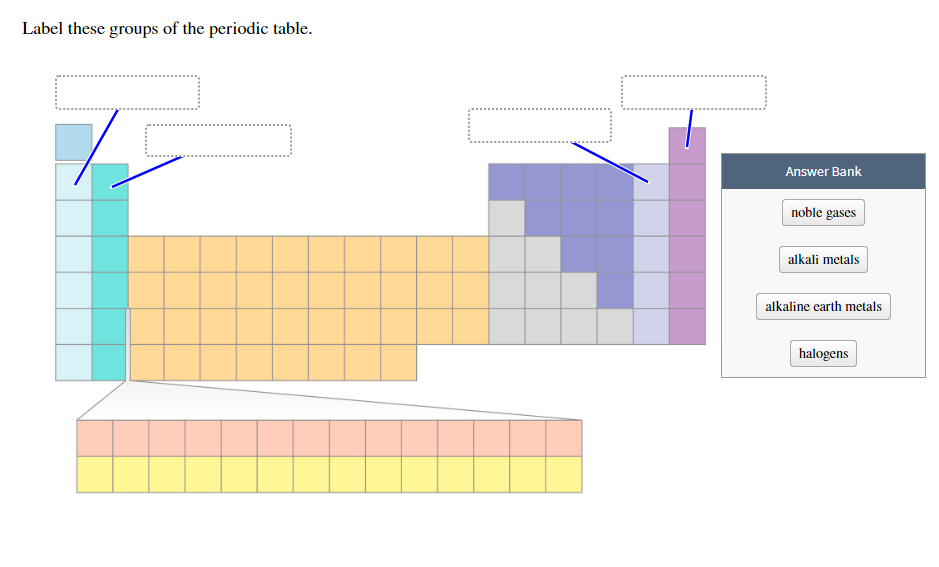
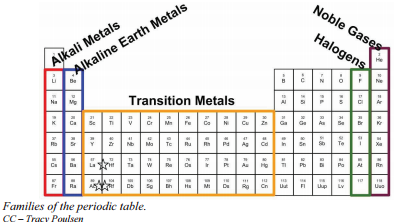
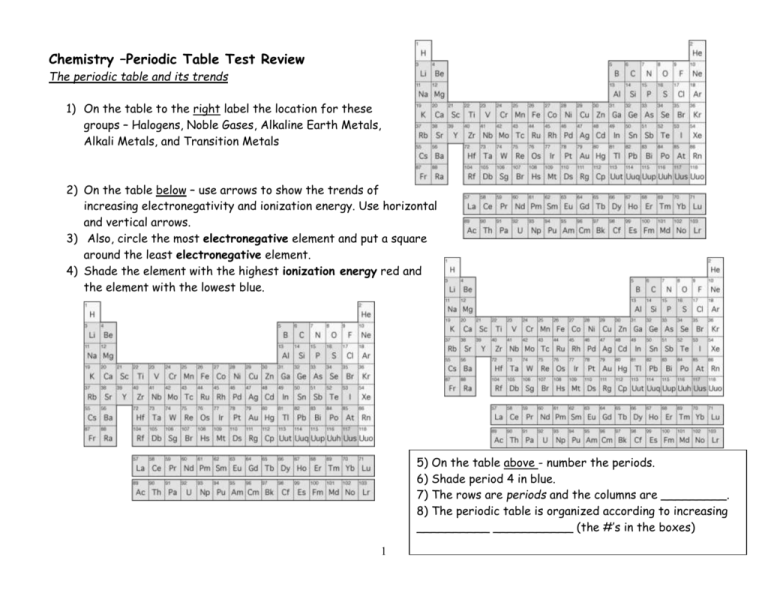
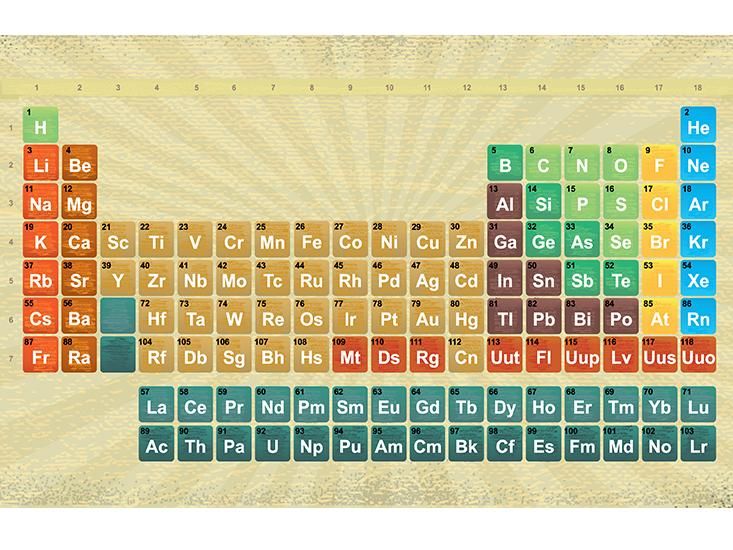
44 label these groups of the periodic table
The modern periodic table - BBC Bitesize The periodic table is a way of organising the elements which is used by scientists to group elements with similar properties. It has a unique arrangement of rows and columns. Noble gas | Definition, Elements, Properties, Characteristics, & Facts noble gas, any of the seven chemical elements that make up Group 18 (VIIIa) of the periodic table. The elements are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), radon (Rn), and oganesson (Og). The noble gases are colourless, odourless, tasteless, nonflammable gases. They traditionally have been labeled Group 0 in the periodic table because for decades after their discovery it ...
Group (periodic table) - Wikipedia In chemistry, a group (also known as a family) is a column of elements in the periodic table of the chemical elements.There are 18 numbered groups in the periodic table; the 14 f-block columns, between groups 2 and 3, are not numbered. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their atoms (i.e., the same core charge), because ...
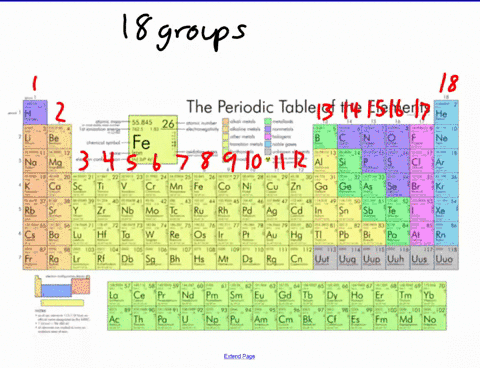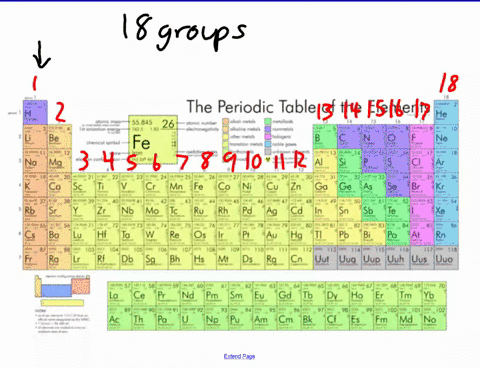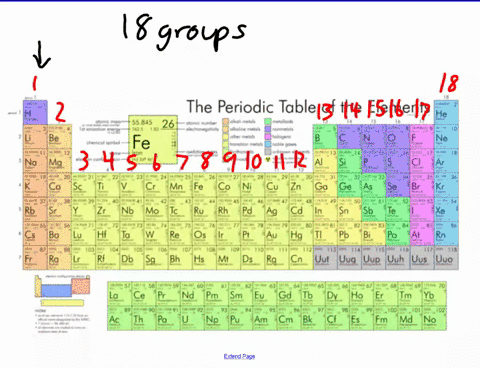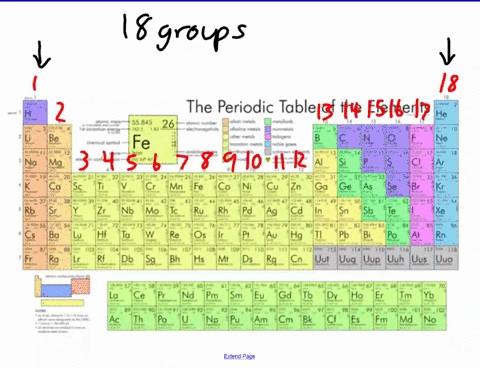
Label these groups of the periodic table
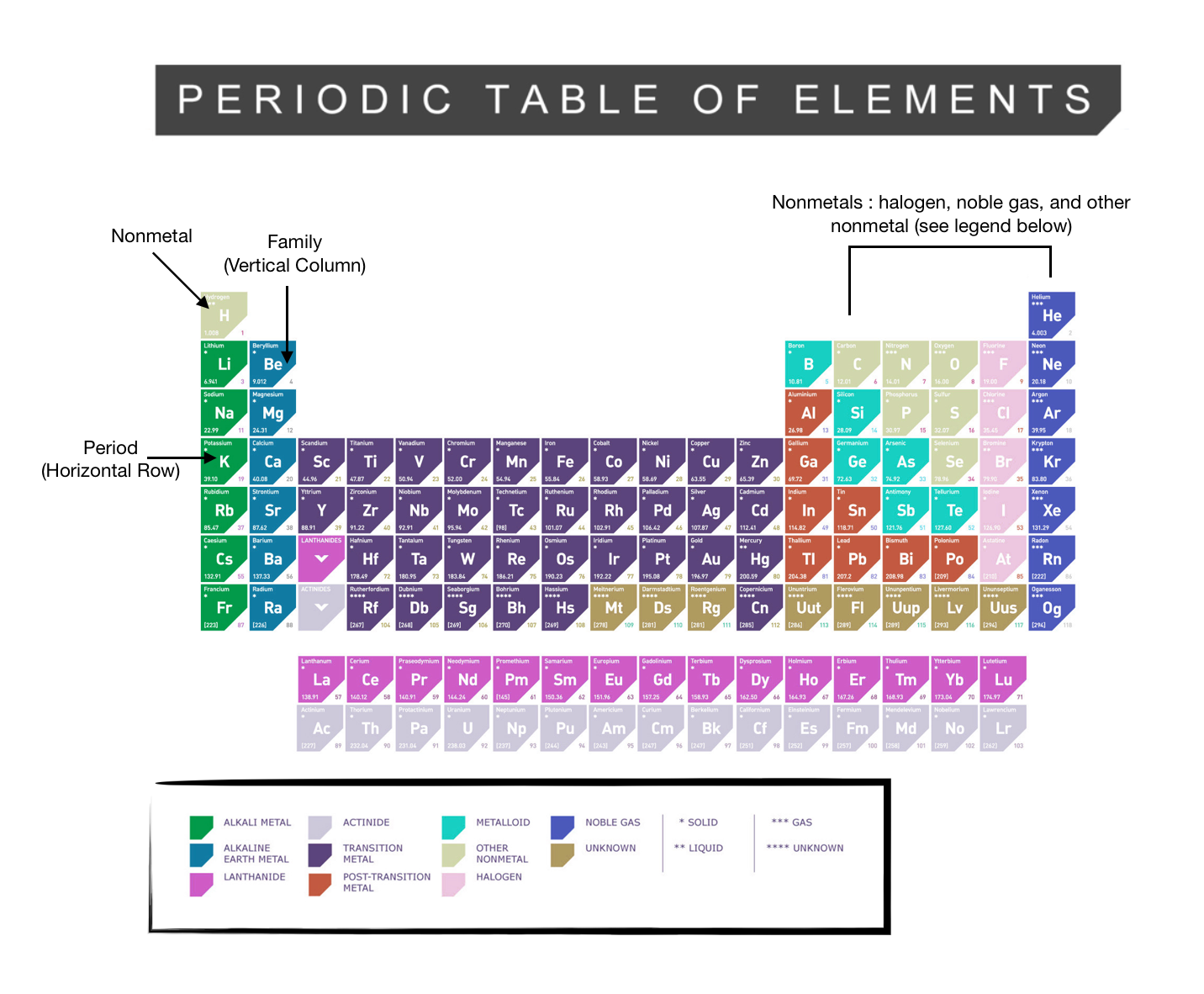
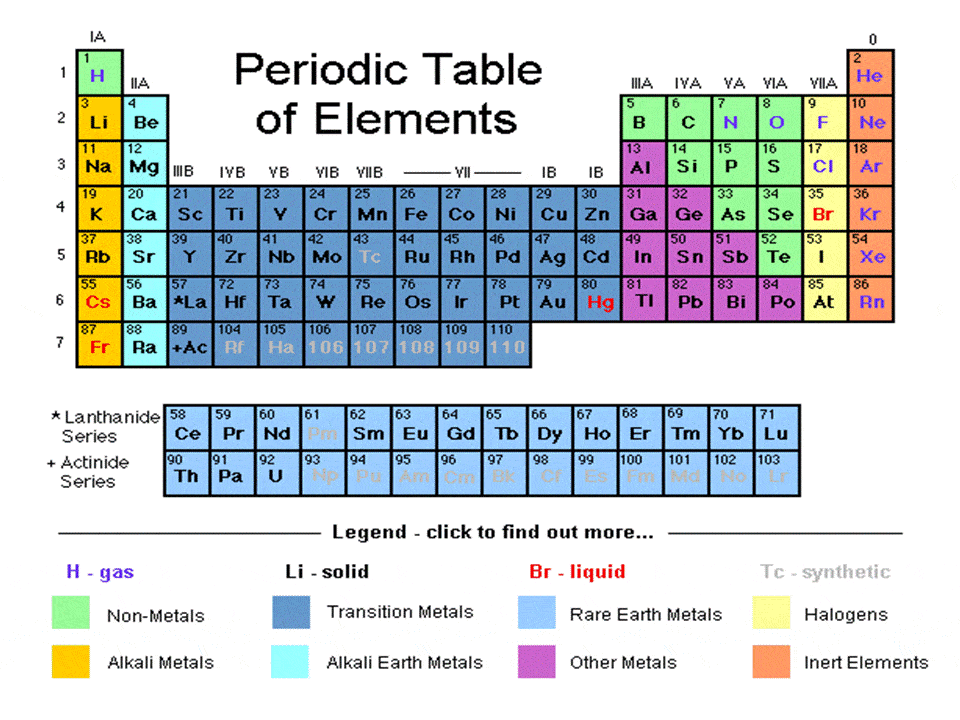
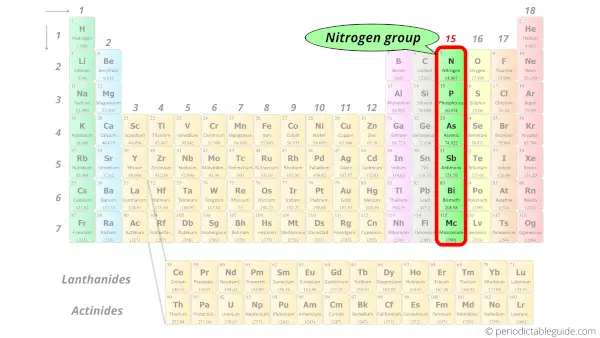
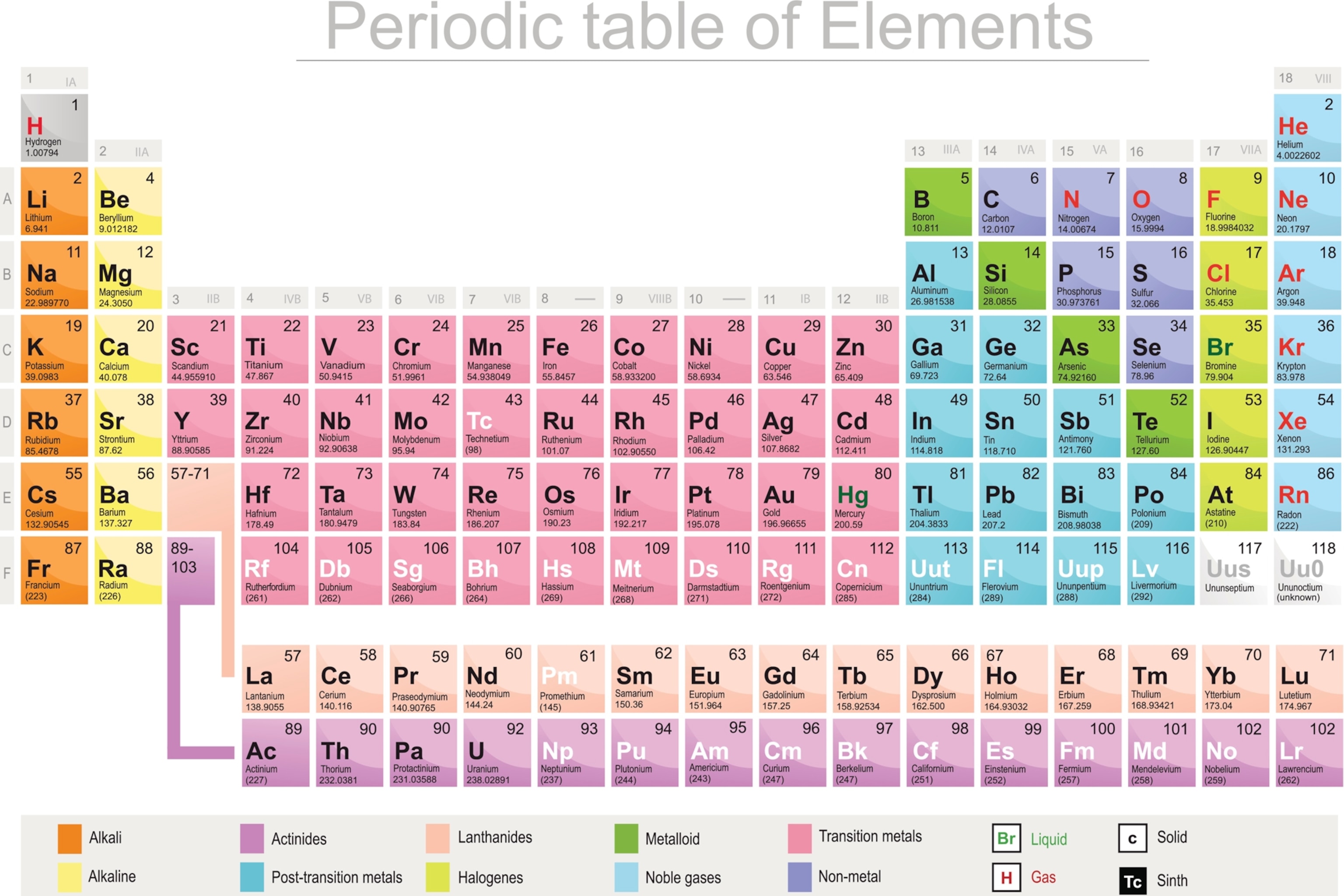
The periodic table, electron shells, and orbitals - Khan Academy By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties.These properties determine an element's physical state at room temperature—gas, solid, or liquid ... 6.4: Modern Periodic Table- Periods and Groups The Modern Periodic Table. The periodic table has undergone extensive changes in the time since it was originally developed by Mendeleev and Moseley. Many new elements have been discovered, while others have been artificially synthesized. Each fits properly into a group of elements with similar properties. The periodic table is an arrangement ... Periodic table Groups Explained !! (With 1-18 Group Names) There are total 18 different groups in Periodic table. Group 1: Alkali metals group (hydrogen not included) Group 2: Alkaline earth metals group. Group 3-12: Transition and Inner transition metals group. Group 13: Boron group. Group 14: Carbon group. Group 15: Nitrogen group.
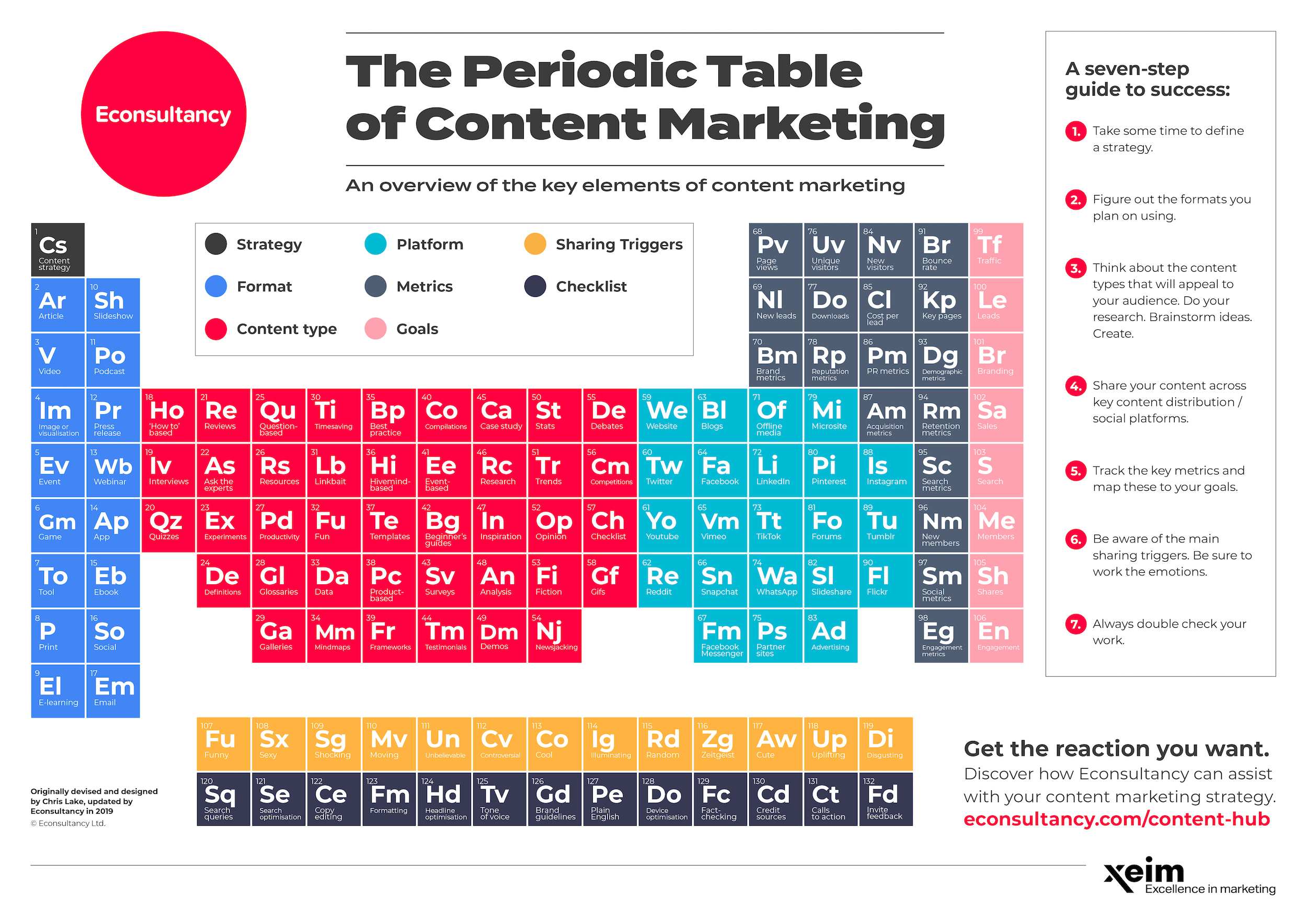
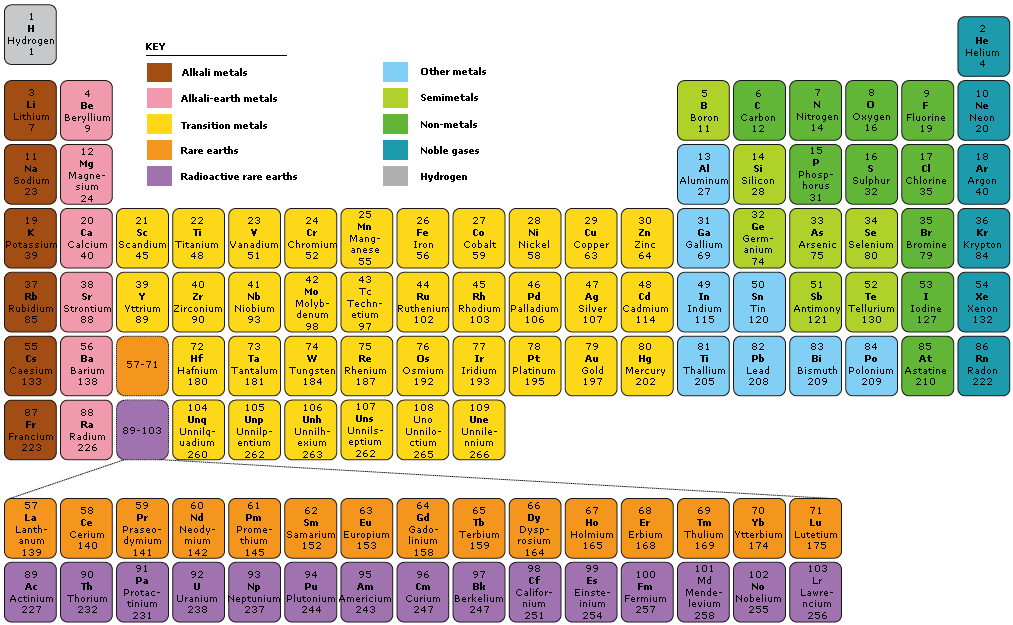
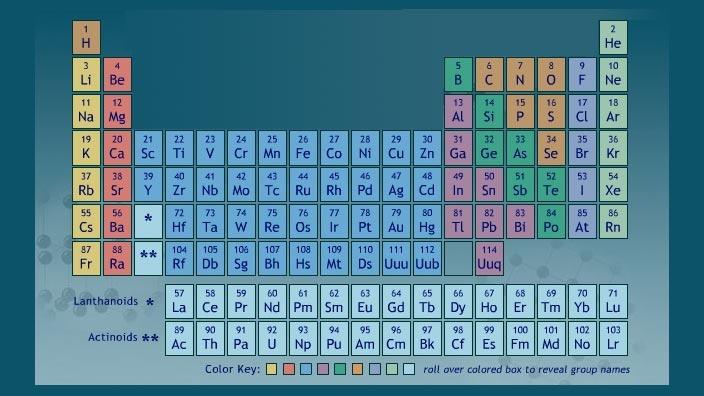
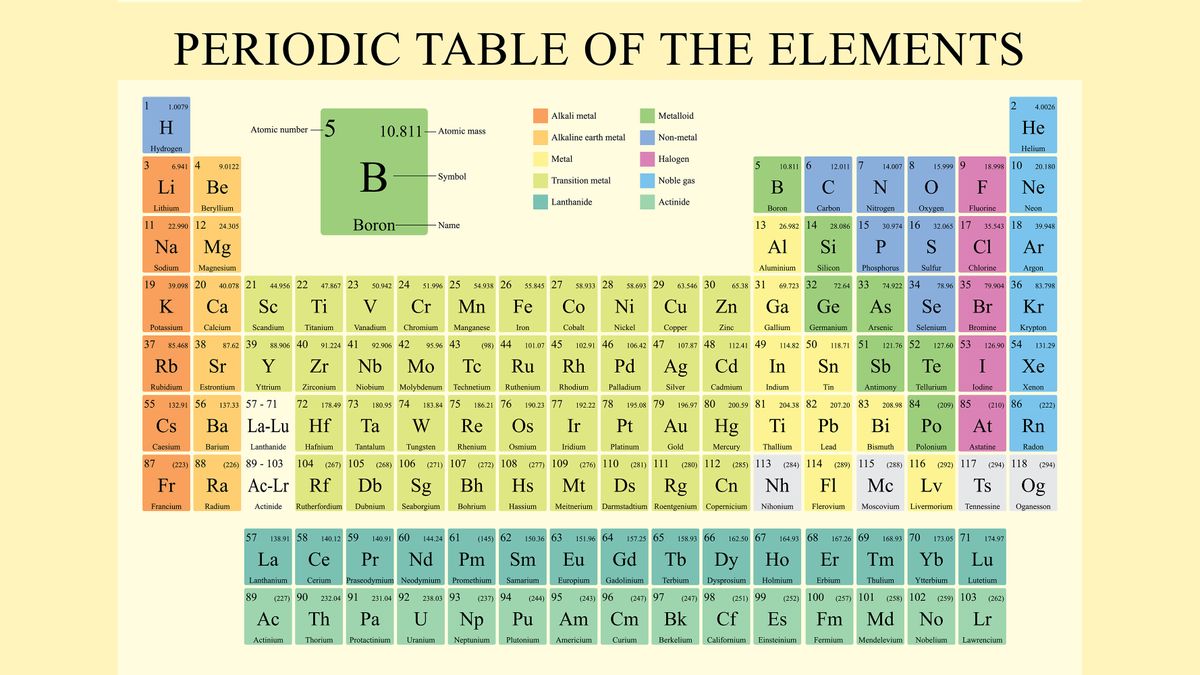
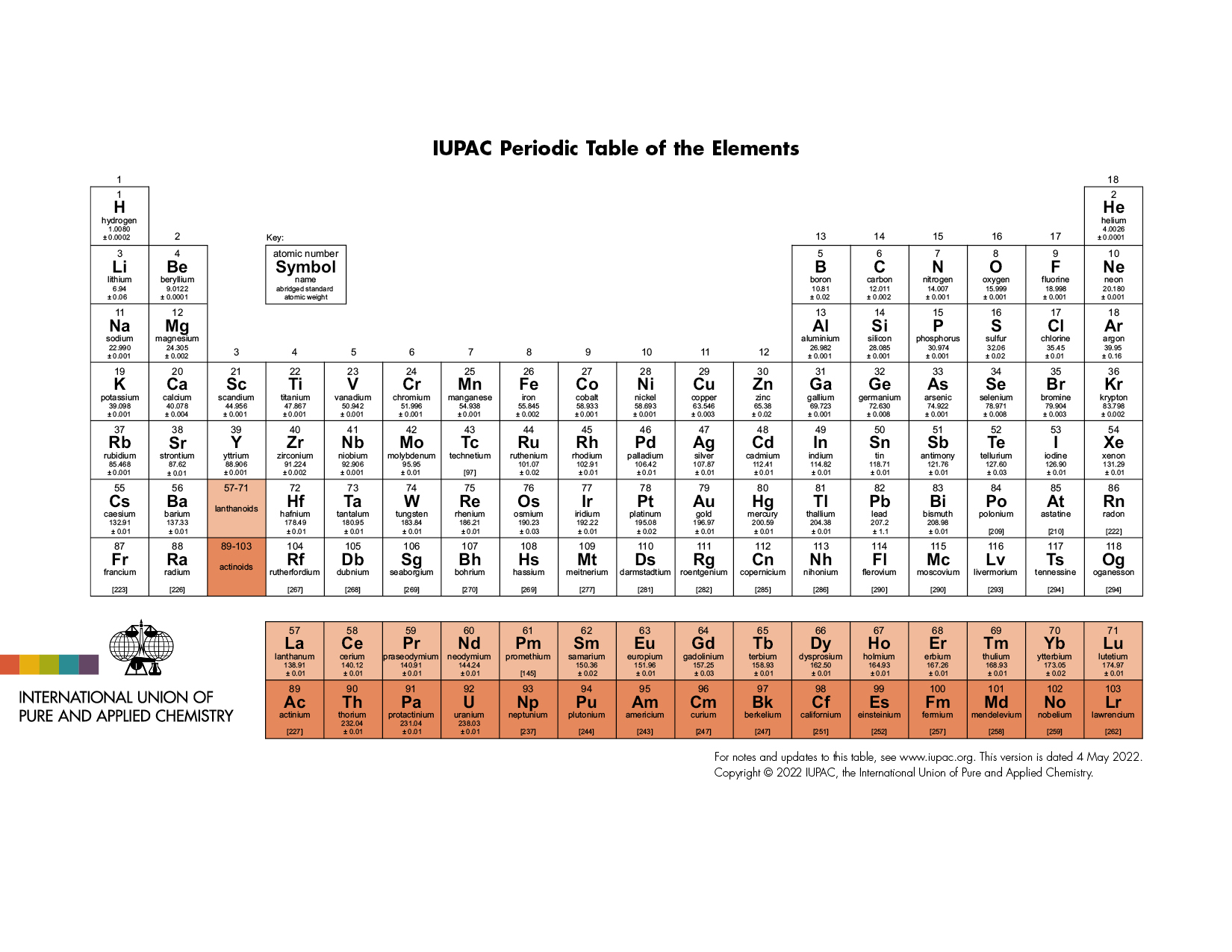
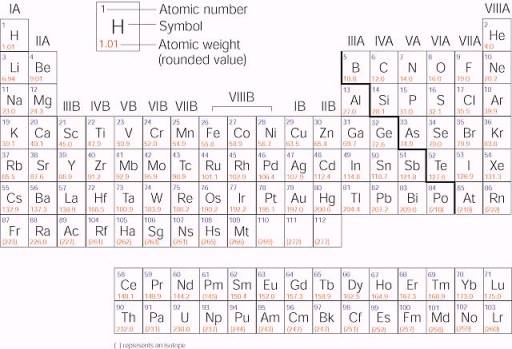
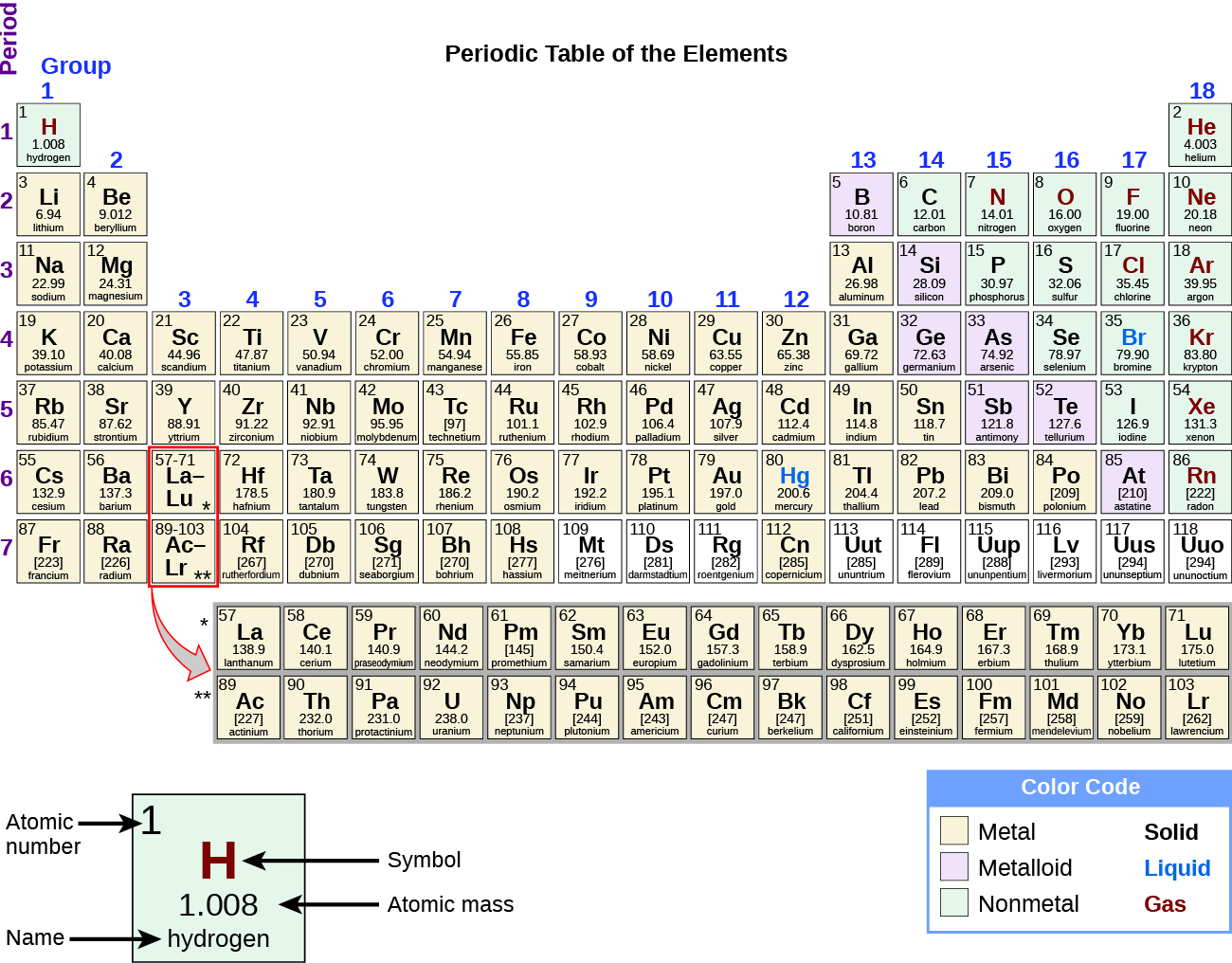
Label these groups of the periodic table. Labeling Periodic Table Teaching Resources | TPT Students will explore the families and groups of the periodic table by creating their own digital reference. Students are given a blank digital periodic table on Google Sheets that they can edit. Students also have the option of completing the assignment on paper.Students will label the following on the periodic table1. Periods2. Groups3. Metals4. How the Periodic Table groups the elements | Live Science Groups of the Periodic table Alkali metals: The alkali metals make up most of Group 1, the table's first column. Shiny and soft enough to cut with a knife, these metals start with lithium (Li) and ... Groups in the periodic table - AQA Synergy - BBC Bitesize Group 1. contains elements. placed in a vertical column on the far left of the periodic table. The elements in group 1 are called the alkali metals . Group 1 is on the left-hand side of the ... 3.2: Organization of the Periodic Table - Chemistry LibreTexts A modern periodic table arranges the elements in increasing order of their atomic numbers and groups atoms with similar properties in the same vertical column (Figure 3.2. 2 ). Each box represents an element and contains its atomic number, symbol, average atomic mass, and (sometimes) name. The elements are arranged in seven horizontal rows ...
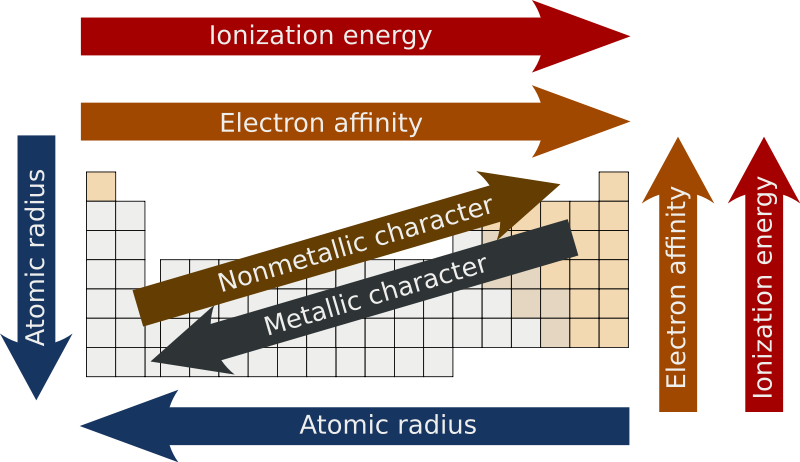
Groups of the periodic table (video) | Khan Academy Groups of the periodic table. The s-, p-, and d-block elements of the periodic table are arranged into 18 numbered columns, or groups. The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity. Created by Sal Khan. Properties of Periodic Table of Element Groups - ThoughtCo Alkali Metals. Less dense than other metals. One loosely bound valence electron. Highly reactive, with reactivity increasing moving down the group. The largest atomic radius of elements in their period. Low ionization energy. Low electronegativity. The periodic table - classification of elements - Khan Academy The groups are the vertical columns on the periodic table. And so, if I go over here, I can see that all of these elements are in the same vertical column. So all these elements are in the same group. And we call this group 1. I can see that all of these elements are also in the same column. Periodic table | Definition, Elements, Groups, Charges, Trends, & Facts ... periodic table, in full periodic table of the elements, in chemistry, the organized array of all the chemical elements in order of increasing atomic number—i.e., the total number of protons in the atomic nucleus. When the chemical elements are thus arranged, there is a recurring pattern called the "periodic law" in their properties, in which elements in the same column (group) have ...
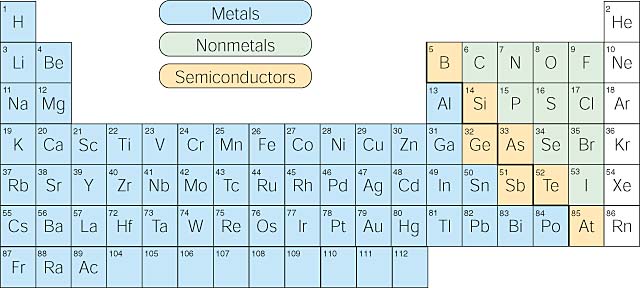
Groups and periods - The periodic table - BBC Bitesize are arranged in the periodic table. Here are the main features of the table: the horizontal rows are called periods; the vertical columns are called groups; elements in the same group are similar ... Solved Label these groups of the periodic table. | Chegg.com Expert Answer. 100% (62 ratings) Transcribed image text: Label these groups of the periodic table. 2.3: Families and Periods of the Periodic Table Vocabulary. Group (family): A vertical column in the periodic table. Alkali metals: Group 1A of the periodic table. Alkaline earth metals: Group 2A of the periodic table. Halogens: Group 7A of the periodic table. Noble gases: Group 8A of the periodic table. Transition elements: Groups 3 to 12 of the periodic table. Carbon group element | chemical elements | Britannica carbon group element, any of the six chemical elements that make up Group 14 (IVa) of the periodic table—namely, carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb), and flerovium (Fl). Except for germanium and the artificially produced flerovium, all of these elements are familiar in daily life either as the pure element or in the form of compounds, although, except for silicon ...
Solved Label these groups of the periodic table. Answer Bank - Chegg Considering the order from left to right, i would like give them numbers as 1,2,3 and 4. So the answer is 1 is Group 1 named as Alkali metals 2 is Group 2 named as Alk …. View the full answer. Transcribed image text: Label these groups of the periodic table. Answer Bank noble gases alkali metals alkaline earth metals halogens.
Periods On A Periodic Table Teaching Resources | TPT Students will explore the families and groups of the periodic table by creating their own digital reference. Students are given a blank digital periodic table on Google Sheets that they can edit. Students also have the option of completing the assignment on paper.Students will label the following on the periodic table1. Periods2. Groups3. Metals4.
Periodic Table of Elements - PubChem Chemical Element Data in PubChem. PubChem is providing this periodic table page in order to help navigate abundant chemical element data available in PubChem. When exploring the table or list views on this page, please note the links to dedicated pages for each element. These individual element summary pages contain a lot of additional ...
Periodic table Groups Explained !! (With 1-18 Group Names) There are total 18 different groups in Periodic table. Group 1: Alkali metals group (hydrogen not included) Group 2: Alkaline earth metals group. Group 3-12: Transition and Inner transition metals group. Group 13: Boron group. Group 14: Carbon group. Group 15: Nitrogen group.
6.4: Modern Periodic Table- Periods and Groups The Modern Periodic Table. The periodic table has undergone extensive changes in the time since it was originally developed by Mendeleev and Moseley. Many new elements have been discovered, while others have been artificially synthesized. Each fits properly into a group of elements with similar properties. The periodic table is an arrangement ...
The periodic table, electron shells, and orbitals - Khan Academy By convention, elements are organized in the periodic table, a structure that captures important patterns in their behavior.Devised by Russian chemist Dmitri Mendeleev (1834-1907) in 1869, the table places elements into columns—groups—and rows—periods—that share certain properties.These properties determine an element's physical state at room temperature—gas, solid, or liquid ...




:max_bytes(150000):strip_icc()/PeriodicTableCrystal-56a12d9b5f9b58b7d0bccfdf.png)




:max_bytes(150000):strip_icc()/periodic-table-165930186-590f2d703df78c92832fe141.jpg)





:max_bytes(150000):strip_icc()/Periodic-Table-Metals-56a12db33df78cf772682c44.png)


Post a Comment for "44 label these groups of the periodic table"