40 labelled diagram of ph scale
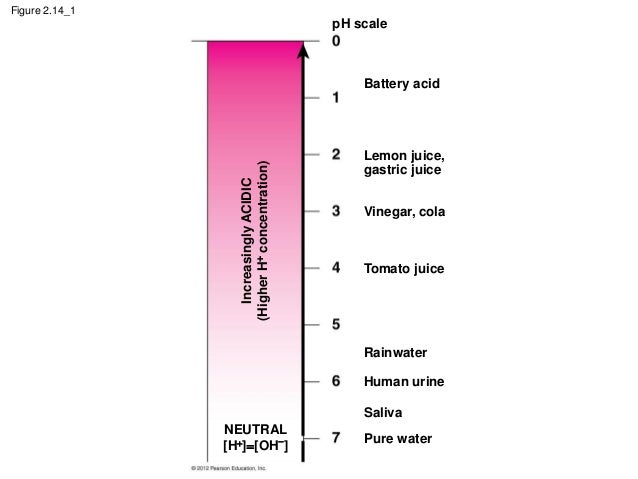
The pH scale with some common examples The pH scale, with examples of common solutions and their pH values. Download/View For commercial use please contact us Label The Ph Scale Diagram / Science Worksheets Label Color The Ph ... The neat and labeled diagram of ph scale is as shown. a diagram a b diagram b c diagram c d diagram d. Ph 7 corresponds to neutral ph. As this diagram shows, ph ranges from 0 to 14, with 7 being neutral. Students then color the given ph scale reflected in ph indicator pap. Find out what negative ph means. If you are given the molarity of hydrogen.
Acids & Bases - pH Scale - Labelled diagram - Wordwall Strong Acid, Moderate Acid, Weak Acid, Neutral, Weak Base, Moderate Base, Strong Base, pH 0-2, pH 3-4, pH 5-6, pH 7, pH 8-9, pH 10-11, pH 12-14, Hydrochloric Acid (HCl), Vinegar (Acetic acid), Lemon juice (Citric Acid), Water, Sodium bicarbonate (baking soda), Ammonia, Sodium hydroxide (NaOH). Acids & Bases - pH Scale Share Share

Labelled diagram of ph scale
Diagram of the pH scale with examples of acidic, neutral and alkaline ... File size: 36.7 MB (381.7 KB Compressed download) Releases: Model - no | Property - no Do I need a release? Dimensions: 4708 x 2724 px | 39.9 x 23.1 cm | 15.7 x 9.1 inches | 300dpi Photographer: Spencer Sutton More information: Diagram of the pH scale with examples of acidic, neutral and alkaline substances. Search stock photos by tags Show all Draw neat and labeled diagram of pH scale? - toppr.com The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH. Was this answer helpful? 0 0 Classes Class 5 Class 6 Class 7 Class 8 Class 9 Class 10 Class 11 Commerce Class 11 Engineering Class 11 Medical Ph Scale With Labels - Science 101 Understanding Ph Sanidepot The neat and labeled diagram of ph scale is as shown. The range of ph is from 0 to 14. Michael heim / eyeem / getty images at 25 c, the ph of pure water is very close to 7. Phs of less than 7 indicate acidity, . Learn more about ph levels of acids and bases. With specific ph values, then draw and label those substances in the worksheet.
Labelled diagram of ph scale. Making a pH indicator using red cabbage - RSC Education 8. Investigate reactions between acids and bases; use indicators and the pH scale; Leaving Certificate. Chemistry. 9. Environmental chemistry: water. 9.1 pH Scale. Depth of treatment. pH scale. Use of universal indicator paper or solution. Limitations of the pH scale - usefulness confined to dilute aqueous solutions. Theory of acid-base ... Chemistry: pH scale Diagram | Quizlet Basic (Alkaline) A solution with a pH above 7. Greater concentration of hydroxide ions than concentration of hydrogen ions. pH scale according to the universal pH indicator strip Red is acidic; green is neutral; blue is basic/alkaline 1M hydrocloric acid (HCl) pH0 1M Sodium Hydroxide (NaH) pH14 Water (H2O) pH7 THIS SET IS OFTEN IN FOLDERS WITH... The pH Scale | Biology for Non-Majors I | | Course Hero The pH scale, which measures from 0 to 14, provides an indication of just how acidic or basic a substance is. Most parts of our body (excluding things like stomach acid) measure around 7.2 and 7.6 on the pH scale (a 7 is neutral on the scale). If foreign strong substances dramatically change this pH, our bodies can no longer function properly. pH Scale - PhET pH Scale - PhET
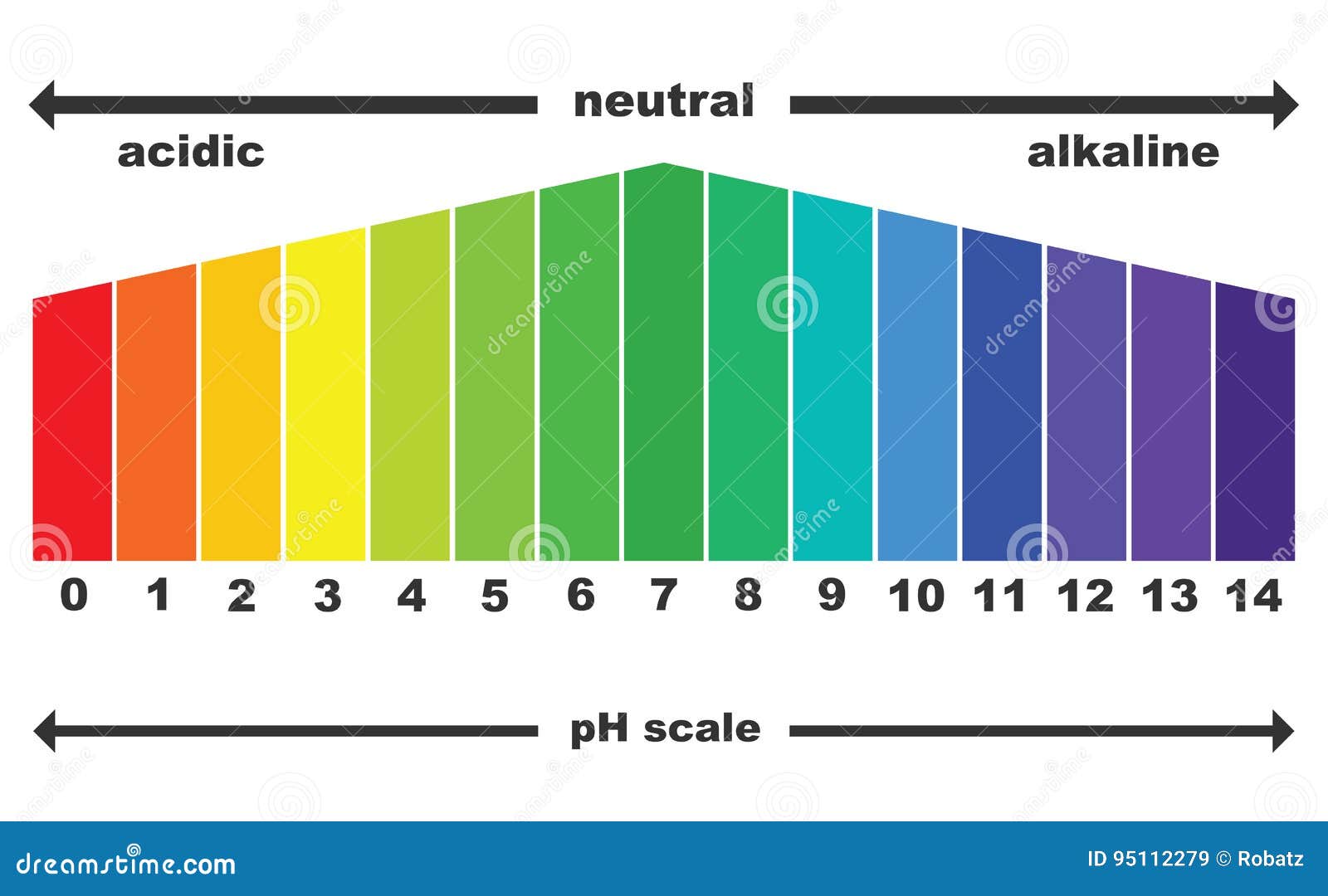
pH - Wikipedia The pH scale is logarithmic and inversely indicates the concentration of hydrogen ions in the solution. where M = mol dm −3. At 25 °C, solutions with a pH less than 7 are acidic, and solutions with a pH greater than 7 are basic. Solutions with a pH of 8 at this temperature are neutral (e.g. pure water ). What is a pH meter and how does it work? - OMEGA pH Meter. pH meter is an instrument used to measure acidity or alkalinity of a solution - also know as pH. pH is the unit of measure that describes the degree of acidity or alkalinity. It is measured on a scale of 0 to 14. The quantitative information provided by the pH value expresses the degree of the activity of an acid or base in terms of ... PH Meter Definition, Principle, Parts, Types, Application, Procedure. Portable pH meter: covering an extensive range of regularly employed instruments, the exception is the use of compact DC power equipment can be produced to the scene. Desktop pH meter: Same as Portable pH meter. Pen pH meter: normally composed of a single scale, conventional measurement range, for the easy and handy equipment. Draw a neat and labelled diagram of pH scale class 10 chemistry CBSE Hint : We all are familiar with the term p H and p H scale. p H scale is a scale of acidity and basicity which tells about the acidic and alkaline behaviour of substance . > On the p H scale the value ranges from 0 to 14. So the more acidic solution will have lower p H and neutral solutions will have p H equal to 7 and more than 7 acidic nature ...
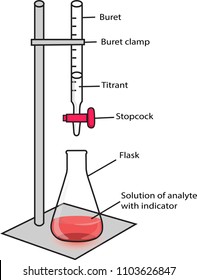
pH Scale | U.S. Geological Survey - USGS.gov Each number represents a 10-fold change in the acidity/basicness of the water. Water with a pH of five is ten times more acidic than water having a pH of six. As this diagram shows, pH ranges from 0 to 14, with 7 being neutral. pHs less than 7 are acidic while pHs greater than 7 are alkaline (basic). Learn more about pH Sources/Usage pH meter principle and working - Laafon Galaxy Pharmaceuticals A pH meter is a scientific instrument that measures the hydrogen-ion activity in solutions, indicating its acidity or basicity (alkalinity) expressed as pH value. The principle of pH meter is the concentration of hydrogen ions in the solution e.g. it is the negative logarithm of an hydrogen ion. The pH range of solutions varies between 1 to 14 ... The pH scale - BBC Bitesize The pH scale is a number scale from 0 to 14. It tells us how acidic or alkaline an aqueous solution is. The pH scale is used to classify as acidic, alkaline or neutral. Neutral solutions are... pH (TITRATION) CURVES - chemguide Simple pH curves. All the following titration curves are based on both acid and alkali having a concentration of 1 mol dm-3.In each case, you start with 25 cm 3 of one of the solutions in the flask, and the other one in a burette.. Although you normally run the acid from a burette into the alkali in a flask, you may need to know about the titration curve for adding it the other way around as well.
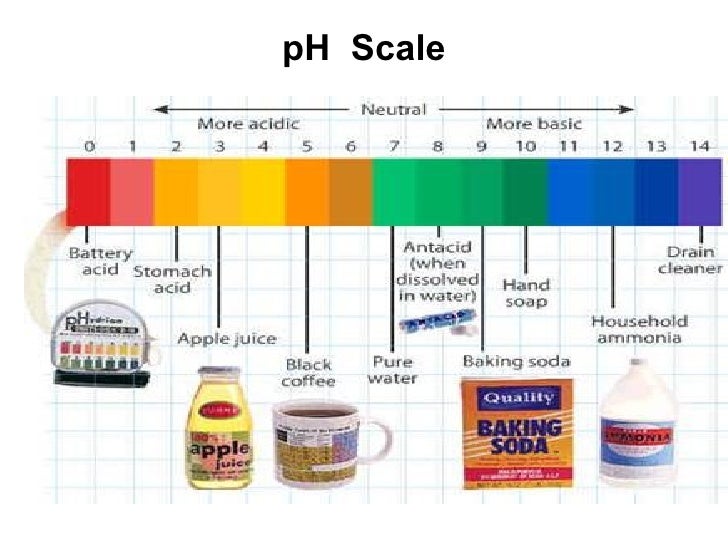
Label The Ph Scale Diagram / Ph Scale Infographic Acidbase Balance ... Start studying label the ph scale. Ph less than 7 corresponds to acidic . As this diagram shows, ph ranges from 0 to 14, with 7 being neutral. A ph of 7 is neutral. Phs less than 7 are acidic while phs greater than 7 are alkaline . Jazzirt/getty images the usual range of ph values runs from 0 to 14.
pH Scale - Labelled diagram Neutral, Weak Acid, Strong Acid, Weak Alkali, Strong Alkali, Stomach Acid, Water, Indigestion Tablets, Drain Cleaner.
Acids, Alkalis, and the pH Scale - Compound Interest A pH of spot on 7 denotes a neutral solution (neither acidic or alkaline). Any pH below 7 is acidic, whilst any pH above 7 is termed alkaline. Water molecules have the chemical formula H 2 O. However, these molecules are capable of splitting up slightly in solution, in H + and OH - (hydroxide) ions.
pH meter - Wikipedia A pH meter is a scientific instrument that measures the hydrogen-ion activity in water-based solutions, indicating its acidity or alkalinity expressed as pH. The pH meter measures the difference in electrical potential between a pH electrode and a reference electrode, and so the pH meter is sometimes referred to as a "potentiometric pH meter". The difference in electrical potential relates to ...
Draw pH scale and label acids alkalis and neutral. - Sarthaks eConnect ... The neat and labeled diagram of pH scale is as shown. The range of pH is from 0 to 14. pH 7 corresponds to neutral pH. pH less than 7 corresponds to acidic pH. pH more than 7 corresponds to alkaline pH.
Acids, Bases, & the pH Scale - Science Buddies pH = −log [H+] The square brackets around the H + automatically mean "concentration" to a chemist. What the equation means is just what we said before: for each 1-unit change in pH, the hydrogen ion concentration changes ten-fold. Pure water has a neutral pH of 7. pH values lower than 7 are acidic, and pH values higher than 7 are alkaline (basic).
pH Scale - Elmhurst University pH Scale: The pH scale, (0 - 14), is the full set of pH numbers which indicate the concentration of H + and OH - ions in water. The diagram on the left gives some relationships which summarizes much of the previous discussion. pH Scale Principle: H+ ion concentration and pH relate inversely. OH- ion concentration and pH relate directly.
Reactions with acids - pH scale and indicators - BBC Bitesize The pH scale is used to measure acidity and alkalinity. When an acid is neutralised, it forms a salt. Part of. Chemistry (Single Science) Acids, alkalis and salts.
Post a Comment for "40 labelled diagram of ph scale"